New research sheds light on neural processing of diverse classes of rewards in mice, with potential implications for understanding substance use disorders in humans.
Drugs like morphine and cocaine fundamentally warp the brain’s reward system—creating the urge to use while, simultaneously, throwing natural urges to eat and drink off-kilter.
Now, researchers have identified, for the first time, a common reward pathway that may serve as a hub for rearranging such fundamental priorities.
The findings appear in Science.
“We’ve known for decades that natural rewards, like food, and drugs can activate the same brain region,” says Jeffrey F. Friedman, a professor at Rockefeller University.
“But what we’ve just learned is that they impact neural activity in strikingly different ways. One of the big takeaways here is that addictive drugs have pathologic effects on these neural pathways, that’s distinct from, say, the physiologic response to eating a meal when you are hungry or drinking a glass of water when you are thirsty.”
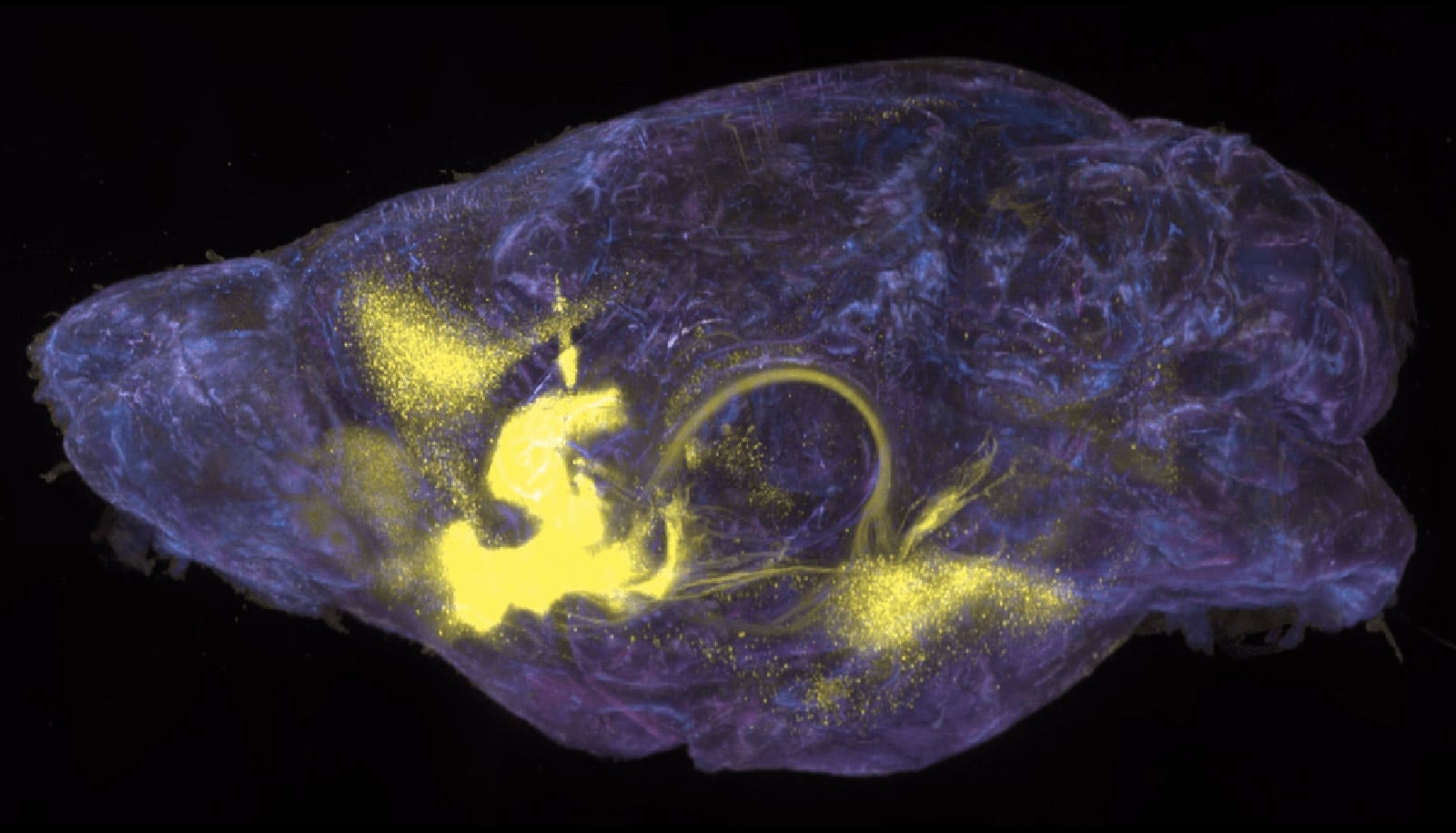
Nestled in the forebrain, the nucleus accumbens (NAc) is involved in processing rewards from and desire for food, sex, social interaction—and addictive substances. The NAc, in close collaboration with the pleasure and mood modulating neurotransmitters dopamine and serotonin, influences decision-making by integrating motivation, reinforcement, and pleasure, essentially encouraging animals to repeatedly pursue activities that routinely feel good.
“The NAc is a key node where the underlying dopaminoceptive neurons direct and refine animals’ behaviors towards their goals,” says Bowen Tan, a graduate student in Friedman’s lab. “What we hadn’t been able to understand is how repeated exposure to drugs corrupts these neurons, resulting in escalated drug-seeking behaviors and a shift away from healthy goals.”
To answer that question, Friedman and Tan teamed up with Mount Sinai’s Eric J. Nestler, a psychiatrist and expert on the molecular neurobiology of drug addiction and depression. Together they turned to Rockefeller’s Alipasha Vaziri to overcome technical limitations that have hampered past work in the field. Brain imaging techniques developed in Vaziri’s lab are among the only tools capable of capturing the majority of the mouse cortex in real-time with high resolution. But in this case, the researchers also needed the capability to record neurons at large tissue depths, in order to image the neural activity at single-cell resolution in the NAc.
“Advancing our understanding of the intricately connected network within the brain demands the innovation of cutting-edge imaging technologies that can capture neuronal activity across distant brain regions, but also that of those at deeper regions,” Vaziri says.
The team ultimately discovered that cocaine and morphine each activate a specific subset of neurons that also respond to natural reward consumption in the mouse NAc. Both cocaine and morphine activated D1 medium spiny neurons in the NAc (involved in positive reinforcement and motivation), while morphine also activated D2 medium spiny neurons (involved in dampening or inhibiting responses to rewarding stimuli).
This cell type-specific response in the NAc was unexpected.
“Though both drugs and natural rewards activate an overlapping set of medium spiney neurons, cocaine and morphine each activate distinct cell types.” Tan says.
“Their distinct actions within the NAc underscore how the diverse neural dynamics elicited by drugs ultimately shape the different behavioral and physiological outcomes observed in regard to natural rewards.”
Then, using a combination of cutting-edge molecular and genomic techniques, including FOS-Seq, CRISPR-perturbation, and snRNAseq, the researchers went on to identify for the first time how drug addiction skews natural urge: by hijacking a molecular pathway that plays a crucial role neural plasticity, the process that neurons use reinforce learning and memory.
When drugs activate neurons expressing this gene, known as Rheb, it stimulates a pathway known as mTOR, likely altering how neurons communicate, learn, and remember stimuli from food and water. This may explain why mice and humans who are addicted to these substances seem to almost forget the need to eat and drink, stimuli that the brain should be reinforcing as rewards.
The team now plans to delve deeper into the cellular biology of addiction neuroscience, in part by identifying how other brain regions work in concert to influence addiction.
“A major part of our ongoing research will be directed to defining how the complex flow of information is incorporated into value computations in brain cells and how that crucial mechanism enables drugs to overtake the processing of natural rewards, leading to addiction,” Nestler says.
Source: Rockefeller University